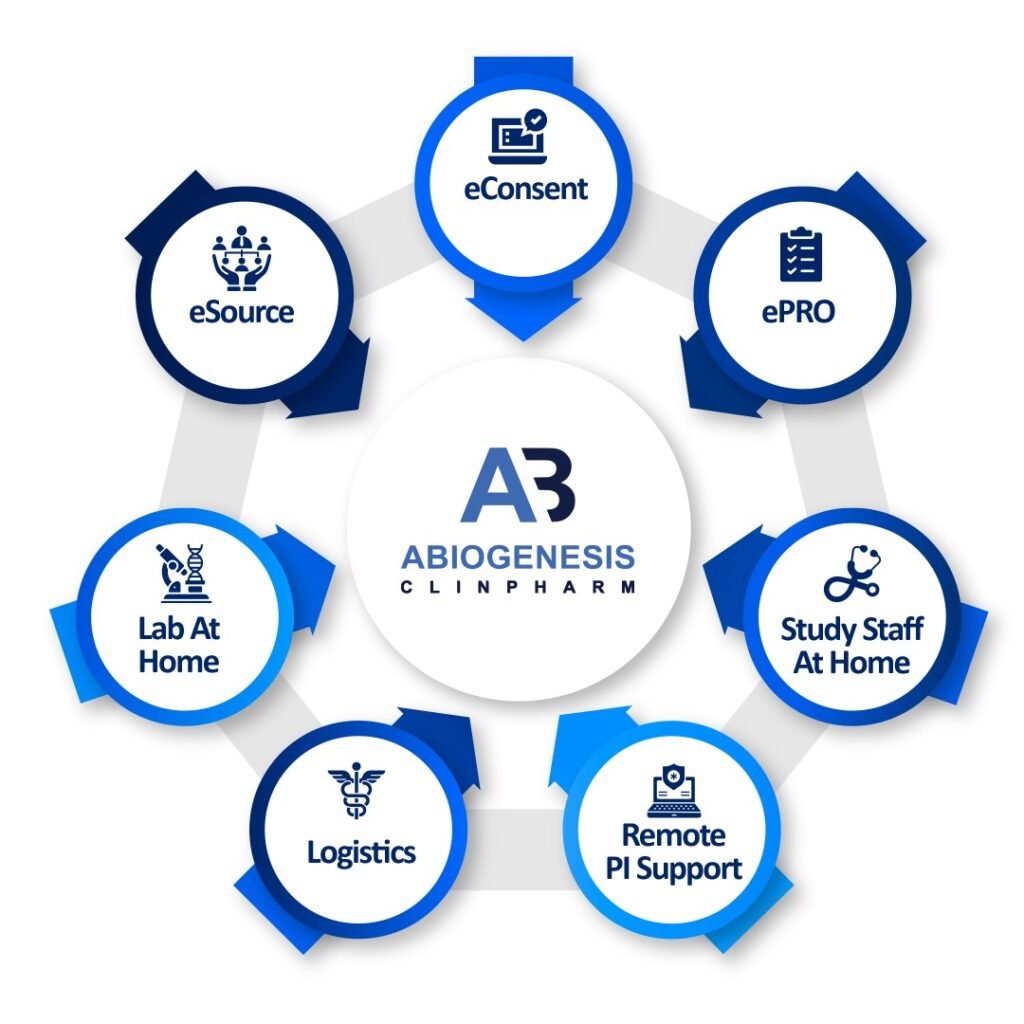
Decentralized clinical trials (DCTs), also known as virtual or remote clinical trials, represent a modern approach to conducting clinical research. These trials aim to improve the efficiency, inclusivity, and patient-centric nature of clinical trials by leveraging technology and reducing the need for patients to visit physical trial sites. Here are some key aspects of decentralized clinical trials where Abiogenesis can help you execute your DCT.
Remote Participation: DCTs enable participants to take part in clinical trials from their homes or other convenient locations.
Technology Utilization: Participants may use smartphones, wearable devices, and other digital tools to collect and transmit data to researchers.
Data Collection: Remote data collection methods, including electronic health records (EHRs), ePROs (electronic patient-reported outcomes), and digital biomarkers, are used to gather essential trial data.
Patient-Centric Approach: DCTs prioritize the convenience and comfort of trial participants by reducing the need for frequent in-person visits.
Inclusivity: Decentralized trials can potentially broaden the participant pool by reaching individuals who may have difficulty accessing traditional trial sites, such as those living in remote areas or with limited mobility.
Enhanced Efficiency: DCTs can accelerate the clinical trial process by reducing administrative and logistical hurdles associated with physical site visits, which can result in faster trial completion and reduced costs.
We as an Abiogenesis are working with many decentralized Clinical Trials where our services are integrated with advanced technologies like CTMS, e-CRF, and e-PRO along with other technological advancements where we can effectively do the DCT along with applicable regulation. If you are looking for decentralized clinical trial services, then please reach out to us for further information.